LabCorp at-home COVID-19 test approved by FDA could be game-changer
FOX 2 - A new at-home test by LabCorp is ready to debut and provide a ground-breaking advancement in the coronavirus fight.
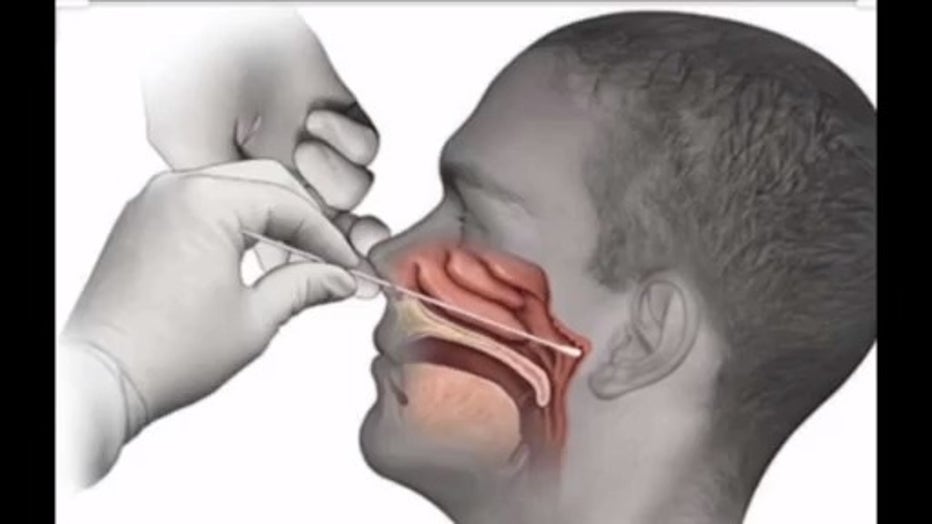
"The swab goes into the nose directly, so it goes into the nose almost until you can touch the back of the throat," said Dr. Sam Allen.

At-home test kits primed to be game-changer in COVID-19 battle
A doctor must prescribe the home collection test kit, which will become available in the next few weeks.
Allen, a critical care doctor who is helping to lead the fight against COVID-19 at Troy Beaumont, described how the new LabCorp at-home test kit just approved by the FDA - is used when you are doing it yourself. He says it is very similar to what his team is doing at the hospital.
"Within the kit is a spiral swab which is like a Q-Tip, but it is a little softer," he said. "What it allows you to do, is take swab back of the nose, twist it and spin it to obtain a specimen. And then it is placed back in the box and sent to the lab for analysis."
A doctor must prescribe the home collection test kit, which will become available in the next few weeks.
The big question everyone is asking, is it accurate? Dr. Allen says yes, if you swab yourself correctly. He believes this test will also be very beneficial when it comes to health care workers.
"Because I would say so because the test is validated and it is a similar test we are using and if you can minimize the amount of PPE and take your healthcare team and minimize the exposure to patients, that may be positive I think that is a great thing," he said.
Allen would know. He was exposed to the coronavirus in March after an emergent procedure to reposition a breathing tube on a COVID-19 patient.
He tested positive for the virus and eventually another test revealed he had the antibodies that allowed him to return to work.
He said it's important to know - the at-home LabCorp test will only test for an active virus.
"Looking at genetic RNA sequence of the virus and it is very similar to what we are doing in the hospital with a nasal swab," he said. "It really tells you the presence or absence of the virus. Remember that it is FDA approved. Some of the tests being done are not FDA approved."
The price is around $100 and will be made available first for health care workers and first responders.


